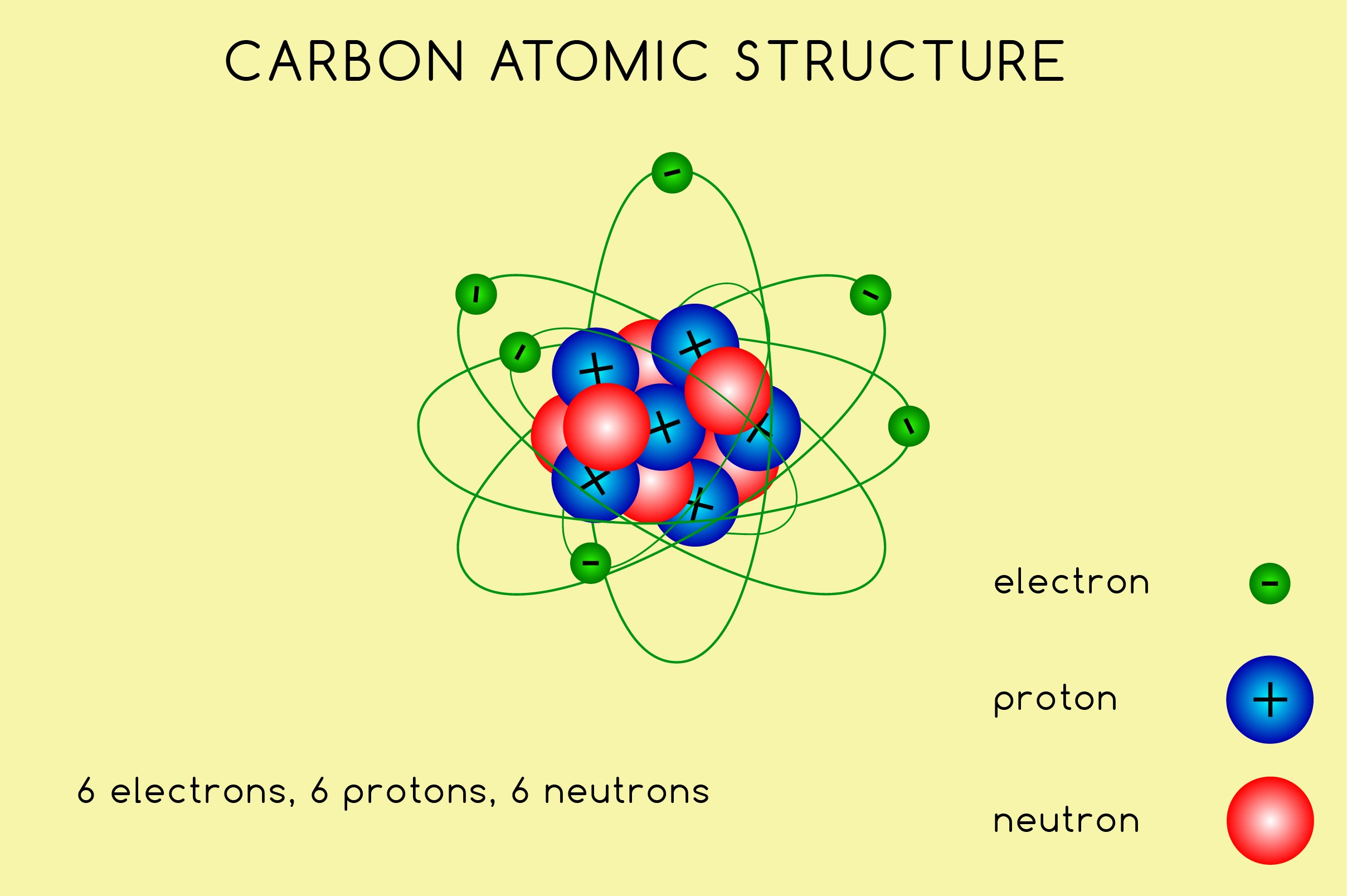
A Carbon Atom Is Most Likely To Form - Carbon most often forms a covalent bond with other atoms. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also. One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. If the bond is with another carbon. As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for. Methane, (\(\ce{ch4}\), is a single carbon atom. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms. To form ionic bonds, carbon molecules must either gain or lose 4 electrons.
The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms. As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. If the bond is with another carbon. One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. Carbon most often forms a covalent bond with other atoms. Methane, (\(\ce{ch4}\), is a single carbon atom.






![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/small/65382287-b5ac-4ea6-8a57-1f5a107b172c/a-carbon-atom---teachoo.jpg)

