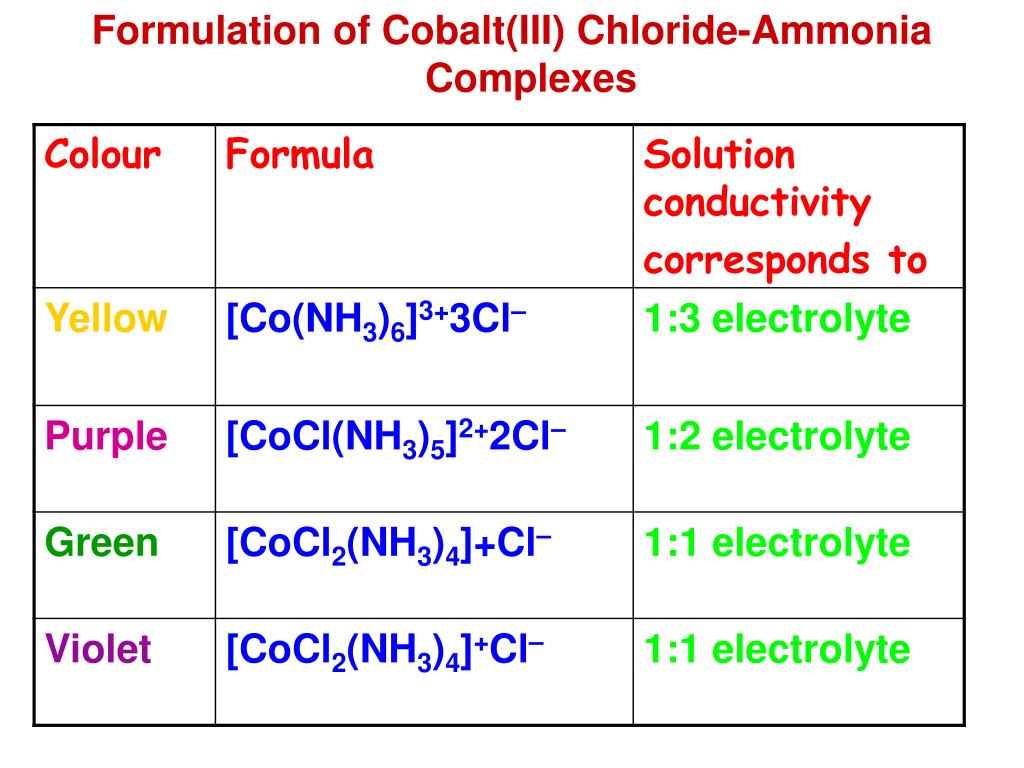
Cobalt Ions Form Complex Ions With Water And Chloride - A ligand exchange reaction involving chloride ions. A solution of cobalt(ii) ion in water is pink, the color of the complex ion formed between co2+ ions and water molecules. Identify the color (either blue, green, yellow, or orange) for the following complex ions formed with \(co^{3+}\): If you add concentrated hydrochloric acid to a solution containing.
Identify the color (either blue, green, yellow, or orange) for the following complex ions formed with \(co^{3+}\): A ligand exchange reaction involving chloride ions. A solution of cobalt(ii) ion in water is pink, the color of the complex ion formed between co2+ ions and water molecules. If you add concentrated hydrochloric acid to a solution containing.